Calculating Average Atomic Mass Quiz / Relative atomic mass questions A level | in 'atomic / An atom has an atomic number of 9 and a mass number of 19.
For example, oxygen has a relative atomic mass of 15.9994 amu. Determine the number of protons present The formula for relative atomic mass is ∑ isotope mass x isotope abundance / 100. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. You use it only when talking about covalent compounds.
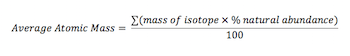
You use it only when talking about covalent compounds.
The relative atomic mass of an element is a. The mole is an important concept for talking about a very large number of things — 6.02 x 10 23 of them to be exact. An atom has an atomic number of 9 and a mass number of 19. The atomic mass can be found underneath the symbol for the element. This module shows how the mole, known as avogadro's number, is key to calculating quantities of atoms and molecules. Relative formula mass (mr) is the same as relative molecular mass. Use a copy of the periodic table of elements.the periodic table lists the atomic mass of each element below the chemical symbol. Calculating relative atomic mass from isotopic abundance. Make sure that you round the atomic mass to the nearest whole number. For example, oxygen has a relative atomic mass of 15.9994 amu. The basics of the atomic theory are that atoms are the smallest units of chemical matter. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. Check your score and answers at the end of the quiz.
The atomic mass can be found underneath the symbol for the element. If a person ingests 7.00 ml of 24.0 mm nacn, what percentage of … You use it only when talking about covalent compounds. The basics of the atomic theory are that atoms are the smallest units of chemical matter. Check your score and answers at the end of the quiz.

Relative formula mass (mr) is the same as relative molecular mass.
This module shows how the mole, known as avogadro's number, is key to calculating quantities of atoms and molecules. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. Use a copy of the periodic table of elements.the periodic table lists the atomic mass of each element below the chemical symbol. Determine the number of protons present You use it only when talking about covalent compounds. The basics of the atomic theory are that atoms are the smallest units of chemical matter. For carbon dioxide (co 2), the relative atomic mass is 12.011 amu for carbon and 15.999 for oxygen. Calculating relative atomic mass from isotopic abundance. The mole is an important concept for talking about a very large number of things — 6.02 x 10 23 of them to be exact. Relative formula mass (mr) is the same as relative molecular mass. An atom has an atomic number of 9 and a mass number of 19. If a person ingests 7.00 ml of 24.0 mm nacn, what percentage of … The relative atomic mass of an element is a.
Make sure that you round the atomic mass to the nearest whole number. For carbon dioxide (co 2), the relative atomic mass is 12.011 amu for carbon and 15.999 for oxygen. This module shows how the mole, known as avogadro's number, is key to calculating quantities of atoms and molecules. The relative atomic mass of an element is a. The basics of the atomic theory are that atoms are the smallest units of chemical matter.

For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11.
If we know the number of protons and the mass number of an element, we can also calculate the number of neutrons simply by subtracting its atomic number from its mass number. Its unit is a unified atomic mass and is denoted by the symbol 'u'. The relative atomic mass of an element is a. The basics of the atomic theory are that atoms are the smallest units of chemical matter. You use it only when talking about covalent compounds. For carbon dioxide (co 2), the relative atomic mass is 12.011 amu for carbon and 15.999 for oxygen. This module shows how the mole, known as avogadro's number, is key to calculating quantities of atoms and molecules. For example, oxygen has a relative atomic mass of 15.9994 amu. If a person ingests 7.00 ml of 24.0 mm nacn, what percentage of … Use a copy of the periodic table of elements.the periodic table lists the atomic mass of each element below the chemical symbol. Calculating relative atomic mass from isotopic abundance. Check your score and answers at the end of the quiz. An atom has an atomic number of 9 and a mass number of 19.
Calculating Average Atomic Mass Quiz / Relative atomic mass questions A level | in 'atomic / An atom has an atomic number of 9 and a mass number of 19.. For carbon dioxide (co 2), the relative atomic mass is 12.011 amu for carbon and 15.999 for oxygen. Check your score and answers at the end of the quiz. Calculating relative atomic mass from isotopic abundance. If a person ingests 7.00 ml of 24.0 mm nacn, what percentage of … Determine the number of protons present
Post a Comment for "Calculating Average Atomic Mass Quiz / Relative atomic mass questions A level | in 'atomic / An atom has an atomic number of 9 and a mass number of 19."